Besrt Describes Best Gas Law Molar Mass
Density mw x P RT Soif P R and T are the same the gas with the greatest molar mass will have the greatest density. M mRT P V M m R T P V.

Gas Laws Equations And Formulas Youtube
The larger the molar mass the larger the density.

. The modified ideal gas law formula. Where m is the mass of the gas and M is the molar mass. To make sure you understand how to find molar mass using the ideal gas law lets work through an example together.
Substitute and rearrangePV massmw RT and mass V mw x P RT and since density mass V. Law of gravity. Which of the following best helps explain why the pressure of a sample of CH 4g molar mass 16gmol.
Questions refer to three gases in identical rigid containers under the conditions given in the table below. Finally putting the equation in terms of molar mass we have. P₁ V₁T₁ P₂ V₂T₂.
The Ideal Gas Law Molar Mass and Density. There are several relationships between the temperature pressure the number of moles and the volume of gases. A beam of neutrons passes through an applied electric field.
The smaller the molar mass the larger the density. Boyles law says at constant temperature the volume and pressure of a sample of gas are inversely proportional V 1P. B The pressure of a gas is directly proportional to the temperature of the gas.
We can plug this into the Ideal Gas Equation. Volume of the gas ml L dm³ m³. V₂ 332 atm 150 L 350 K298 K 500 atm.
Which one of the following best describes Charless Law. The smaller the molar mass the larger the density. LatexnfracmMlatex where m is the mass of the gas and M is the molar mass.
A The volume of a gas is directly proportional to the temperature of the gas. V₂ P₁ V₁ T₂T₁ P₂. A The smaller the molar mass the larger the density.
The larger the molar mass the smaller the. Calculating Molar Mass using the Ideal Gas Equation. P1V1 P2V2 T1.
The molecular masses of the gases because the gas molecules have the same average kinetic energy and mass can be calculated using the equation KEavg12mv2KEavg12mv2. Law of conservation of mass D. Moles Pressure Volume 00821 Temperature If you want to work it out yourself without the molar mass of gas calculator be careful with the units.
In this example well have 1200 ml of nitrogen gas with a. Our gas law calculator uses the following equations. P V m MRT P V m M R T.
Gas particles are packed closely together but have some ability to move. According to ideal gas law PVnRT n massmolar mass PV mMRT ----- PM mVRT ----- PM dRT Including compressibility factor Z we get PM ZdRT at 473K and 1 atm Z PMdRT 1atm18gmmol 0804gmL View the full answer. Mass not required for number of moles calculations.
The ideal gas law best describes the properties of which of the following gases at 0C and 1 atm. What happens to the path of. CH 4 is 22400 cm 3 and its molar mass is 16 grams then how many grams of methane are there in 112 cm 3 of methane gas.
B The larger the molar mass the. The molar mass of an ideal gas can be determined using yet another derivation of the Ideal Gas Law. The larger the.
Which one of the following statements best describes Boyles Law. P V RT m M P V R T m M. Charles law says at constant pressure the volume and temperature of a sample of gas.
Which of these statements best describes the relationship between the molar mass and density of a gas. We can plug this into the Ideal Gas Equation. We can write n number of moles as follows.
What is the molecular formula of a compound with a molar mass of 29666 gmol and the empirical formula C 5 H 10 NS 2. For a fixed mass of gas at a constant temperature the pressure of the gas is inversely proportional to its volume. Which of these statements best describes the relationship between the molar mass and density of a gas.

Gas Stoichiometry Problems Youtube
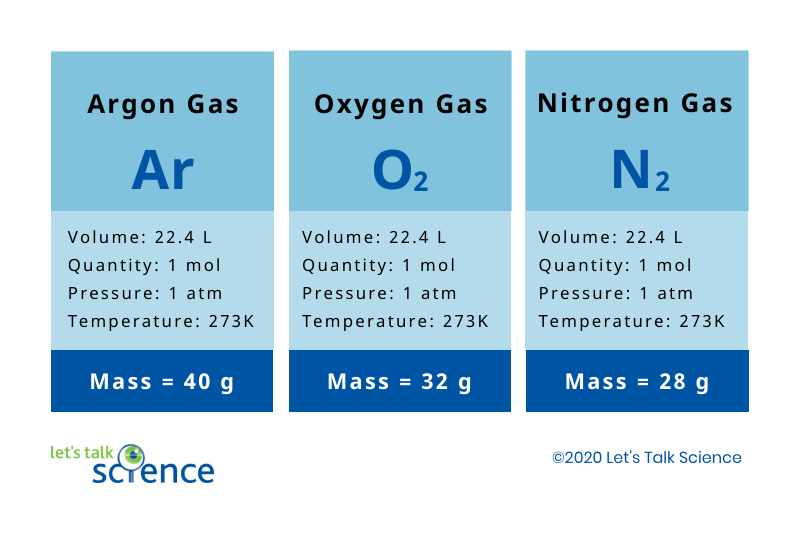
Avogadro And The Ideal Gas Law Let S Talk Science

Gas Density And Molar Mass Formula Examples And Practice Problems Youtube
No comments for "Besrt Describes Best Gas Law Molar Mass"
Post a Comment